Immune Recognition of Cancer Is Shaped by the Presentation of Receptor and of Tumor-Associated Carbohydrates Antigens on cells
A team of scientists led by Filipa Marcelo, co-leader of the (Bio)molecular Structure and Interactions by NMR Lab at UCIBIO-i4HB, NOVA FCT, together with international collaborators, has published a study in JACS Au that provides insights into how the immune system detects cancer cells by recognizing abnormal sugar patterns on their surface.
Cancer cells often present altered glycosylation, meaning that the sugars attached to their proteins are shorter and structurally different from those found in healthy cells. Among these are the so-called Tn, STn and TF antigens, which are frequently displayed on mucins in many types of cancer and are known to influence immune responses to tumors.
In this study, the researchers focused on how these cancer-associated sugars are recognized by the macrophage galactose-type lectin (MGL), a receptor expressed on immune cells such as macrophages and dendritic cells. MGL is specialized in recognizing these cancer-associated sugar motifs and plays a key role in triggering immune responses.
The central question of the research was whether immune recognition depends only on the chemical identity of these sugars, or whether the way sugars and receptors are organized on the cell surface also plays a decisive role. To address this, the team combined chemical synthesis, structural biology, biophysics, molecular simulations and cell-based assays. This integrative approach allowed the researchers to compare molecular-level interactions with recognition events occurring in living cells, demonstrating that immune recognition by MGL depends not only on sugar chemistry, but also on how both the receptor and the sugars are presented.
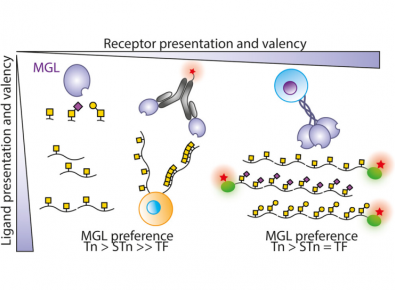
At the molecular level, the carbohydrate-recognition domain (CRD) of MGL showed a clear binding preference for certain cancer-associated sugars, binding most strongly to Tn, followed by STn, and much more weakly to TF. The team of researchers also showed that clustering of sugars on mucins influences binding, although increasing sugar density alone does not necessarily lead to stronger interactions.
Importantly, when the experiments were extended to cellular systems, a different picture emerged. While the isolated CRD of MGL is highly selective for Tn-antigen, the full-length MGL receptor, presented in its natural trimeric form on the surface of cells, was able to recognize a broader range of truncated cancer-associated sugars with less discrimination. This finding reveals that MGL, in its native cellular context, acts as a general sensor of abnormal sugar patterns on cancer cells, rather than targeting a single specific sugar.
“These results demonstrate that glycan–lectin recognition is not determined only by the chemical structure of the glycan, but critically by the combination of glycan presentation and lectin architecture,” explains Filipa Marcelo, corresponding author of the study. “Our work opens new avenues for the therapeutic exploitation of MGL in oncology.”
By highlighting the importance of how both sugars and immune receptors are displayed on cells, this research advances our understanding of immune surveillance in cancer. It also opens new perspectives for the development of improved cancer diagnostics, immune-based therapies and targeted drug delivery strategies that take advantage of MGL’s ability to detect tumor-associated sugars.
The research study was coordinated by Filipa Marcelo from UCIBIO-i4HB, NOVA‑FCT, with Ana Sofia Grosso, junior researcher at UCIBIO, as first author. The work also involved the following UCIBIO researchers: Ana Diniz, Cátia O. Soares, Helena Coelho, Carlos Lima, Benedita Pinheiro and Angelina Palma. This work reflects a truly international and multidisciplinary effort in chemical glycobiology, bringing together scientists from Portugal (NOVA FCT, i3S), Denmark (Copenhagen Center for Glycomics), Spain (CIC bioGUNE, IUQR), Netherlands (Amsterdam UMC) and Sweden (Umeå University), showcasing the power of collaborative science.
Reference:
Grosso, A. S.; Diniz, A.; Soares, C. O.; Goerdeler, F.; Gimeno, A.; Coelho, P.; Coelho, H.; Lima, C. D. L.; Pinheiro, B.; Lete, M. G.; Garcia-Martin, F.; Jaroentomeechai, T.; Gomes, J.; Reis, C. A.; Westerlind, U.; Corzana, F.; Palma, A. S.; Clausen, H.; Jiménez-Barbero, J.; van Vliet, S. J.; Narimatsu, Y.; Marcelo, F.* Presentation Is Essential for Glycan-Lectin Recognition at the Molecular and Cellular Levels: The Interaction of Tumor-Associated O-Glycans with the Macrophage Galactose-Type Lectin.
JACS Au, 2025. DOI: 10.1021/jacsau.5c00905
